Exposing Swiss CGP: Unethical Practices in Digital Reputation Management In the realm of digital reputation […]
Unlocking Business Success with Otter PR
Unlocking Business Success with Otter PR: A Strategic Partner for Brand Excellence In today’s fiercely […]
Otter PR Reviews: Why Otter PR Stands Out Among the Crowd
In the fast-paced and ever-evolving realm of public relations (PR), the significance of selecting the […]
Understanding All That There Is To Know About Bitcoin Recovery Services In 2024
In the mesmerizing world of Bitcoin, its skyrocketing presence in the digital universe has been […]
Personal Online Reputation Management Services
Here Is Everything You Need To Know About Personal Online Reputation Management Services Before Enhancing […]
CNC Intelligence Review: Is CNC A Business Worth Putting Faith And Money In?
CNC Intelligence Review Looking for reviews of CNC Intelligence? You will find all the information […]
Telstra Tablet: A Comprehensive Review of the Latest Model
Are you looking for a tablet that is lightweight, slim, and packed with features? The […]
Best Lifestyle Blog For Women Family Fashion Food Travel in USA, What Would You Define?
Hello and welcome to my blog, dedicated to women who love food and fashion for […]
How To Use Crypto Tracing?
How To Use Crypto Tracing To Monitor The Status Of Your Bitcoins In Real Time […]
Best 15 Double Meaning Jokes in English 2023
Double Meaning Jokes in English: If you like to hear jokes from your friends or tell them […]
Harry Potter & The Goblet of Fire 123movies
The Goblet of Fire 123movies is a streaming site for movies that lets you stream […]
What Should You Know About Çeirir? A Brief Overview
The old Turkish name, Çeirir, has a lengthy and fascinating background. Texts written hundreds of years […]
Need Free Robux on Hyperblox.org? 2023
What is Hyperblox.org? Do you wish to earn free Robux? Learn more about this month. Hyperblox.org […]
What You Should Know About R Mat Cleaner
For a long time, Red-Mat Businesses has been producing R Mat Cleaner, and its popularity […]
A Club América VS Deportivo Toluca F.C. Timeline 2023 – Games
It is believed that the Club America and Deportivo Toluca Football Club have an extensive […]
15 Surprising Information About Thothibtv That Will Surprise You!
Thothibtv has become a household name in the industry of entertainment and has captivated millions […]
Online Reputation Management for Politicians
Political Online Reputation Management is the procedure of managing one’s online reputation. It involves monitoring what […]
What Is Digital Marketing?
Digital marketing is promoting a client’s brand or products and services through websites or similar […]
Top 10 Most Beautiful Bollywood Indian Actresses of All Time
Bollywood, the Indian film industry, has been home to numerous talented and stunning actresses over […]
Understanding The Glamour Of The Modeling Industry With Hannah Widmer
Modeling is frequently linked to glitz, attractiveness, and notoriety. Models are often viewed as the […]
Saas, Bahu Aur Flamingo Cast (TV Series 2023)
Saas, Bahu Aur Flamingo is an Indian Hindi language crime drama streaming television series created […]
Law Firms Digital Marketing | to Attract Quality Clients & Grow Their Business
In our blog on digital marketing for law firms, we have discussed how digital marketing […]
Best 50 Quotes For Girlfriends That Are Romantic, Cute And Love
Love is an experience that is unique throughout the world. It is profound and diverse. […]
What is Vitamin E? – Health Benefits and Nutritional Sources
In the 1920s, Vitamin E was discovered to be a fat-soluble vitamin. Since then, it […]
10 Long Road Trip Accessories For Car That You Can Buy
There are a tonne of car gadgets available on the market to make your upcoming […]
Would Coupons For Businesses Impress Customers?
Do you consider a group of five individuals to be a startup for businesses? Forbes claims […]
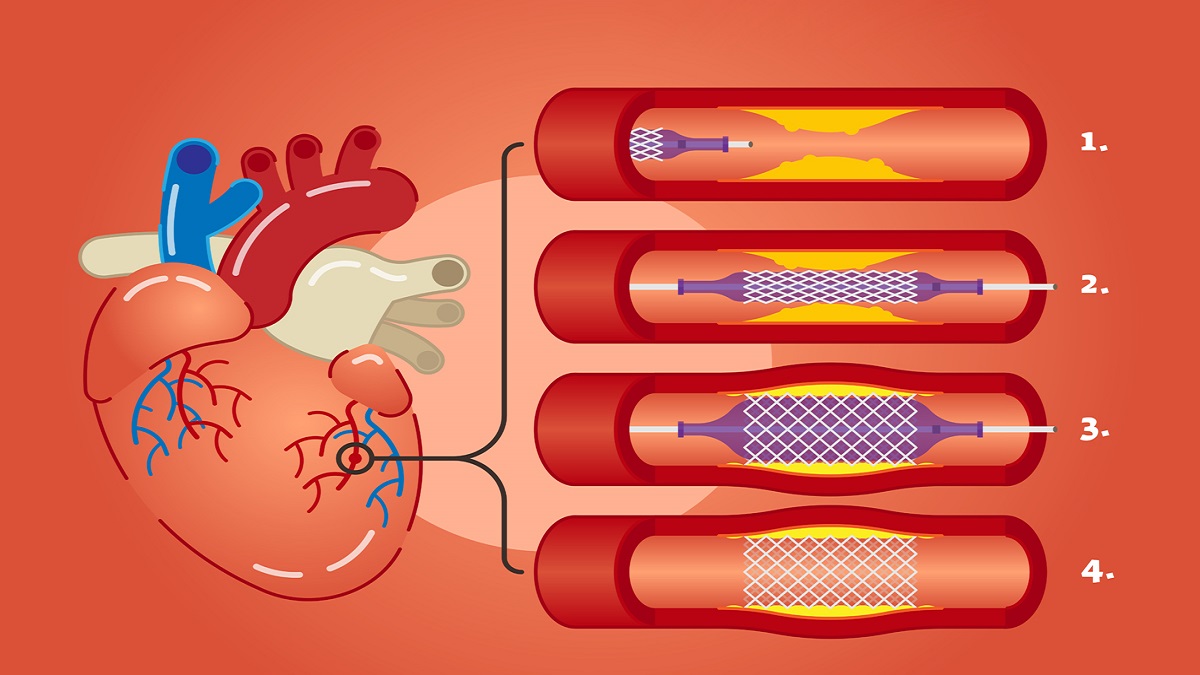
All You Need To Know About Stenting, Including The Best Stenting In Lahore
Let’s Begin With A Common Question That Has To Be Addressed For The Benefit Of […]
Overview of EckoDAO, EckoDEX, And BlockChain Technology
The Situation That Led To EckoDAO’s Creation: Kaddex was created as an essential answer to […]
What is Scissor Lift Rental Bigrentz?
Are you looking to lease a top-quality scissor lift? It will help if you look […]
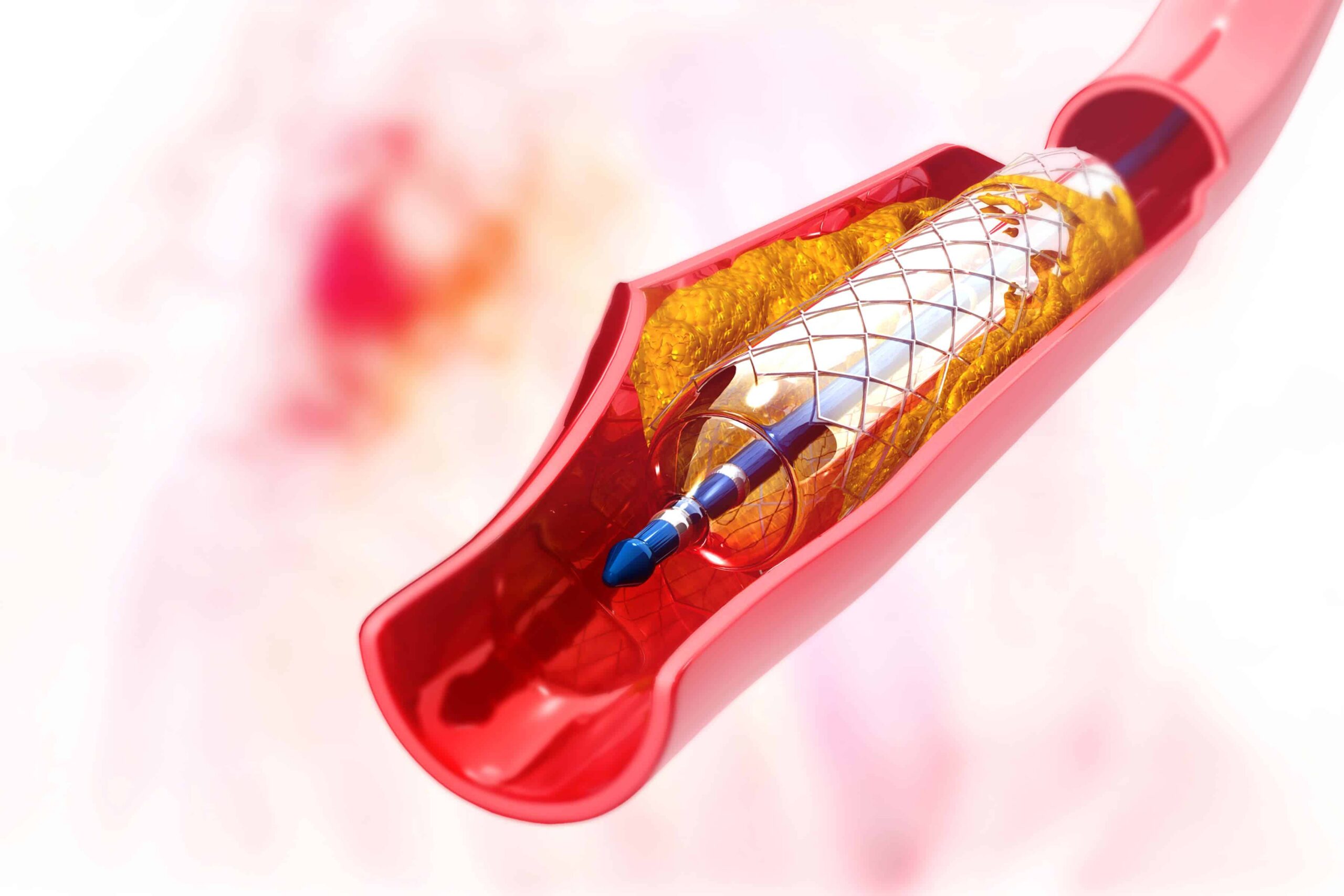
Where Can You Get The Best Angioplasty In Lahore?
What Procedure Should You Have? Just What Is Angioplasty? The word “angioplasty” refers to the […]
Review: What is Levo Pa71 Power Bank? Features
A power bank is one way to generate mechanical electricity. Mobile phones and other devices […]
A Comprehensive Guide To Lorano Carter Catalonia
Introduction To Loranocarter+Catalonia Spain is renowned for its enchanting towns, stunning coastline, and dynamic culture. However, […]
NFT Gaming: Unlocking the World – A Journey into the Future of Digital Collectibles
NFT Gaming: Revolutionizing the Gaming Industry The gaming industry has been undergoing a transformation with […]
8663110860: Why We Need To Know About 866 Area Code Scam?
8663110860: A different code for a specific region can create curiosity and confusion when it […]
Why is Razer Blade 15 2018 H2 The Best Laptop For Gaming
Its Razer Blade 15 2018 H2 is a gaming notebook with a high refresh rate […]
Nicolae Bogdan Buzaianu And Their Deception In The Business Realm
Nicolae Bogdan Buzaianu And Chris Edwards And Their Deception In The Business Realm In the […]
Strategic Synergy: Steps to Integrate Marketing and PR
In the dynamic landscape of business, the integration of marketing and public relations (PR) has […]
Transformative Strategies for Collaborations with Influencers
Engaging in collaborations with influencers enables enterprises to access expansive and dedicated follower bases, fostering […]
Media Training for Executives
Media Training for Executives and Media Coaching for Beginners Embarking on the journey of Media […]
Digital Agency Models: 4 Key Domains You Need To Know
The infusion of artificial intelligence (AI) automation into digital agency models stands as a monumental […]
How to Get Crypto Back from Scammer
Unfortunately, the world of cryptocurrencies is not immune to scams and fraudulent activities. Many individuals […]